Research
Our Three Main Areas of Research
Mechanisms of protection from noise-induced hearing loss
The cellular and molecular bases underlying noise-induced hearing loss (NIHL), the second leading cause of hearing loss globally, are to date, not understood presenting a barrier to the prediction of risk, the prevention, and ultimately the treatment of this debilitating disease. 1.1 billion young people (aged between 12-35 years) are at risk of hearing loss due to exposure to noise in recreational settings. Among Service Members of Operation Enduring Freedom and Iraqi Freedom, NIHL and its associated tinnitus are the top two diagnoses and unaddressed hearing loss poses an annual global cost of $750 billion US dollars. Noise attenuation and hearing aids currently represent the only measures for protection and treatment, respectively. It is now clear that cochlear synaptic loss precedes hair cell loss at low-moderate noise exposures (nonexplosive) effectively silencing affected neurons. Our laboratory and others have illuminated genetic mechanisms that modify sensitivity to NIHL in mice and humans. Through mouse GWAS we have identified a critical gene, Prkag2 encoding the g2 subunit of the AMPK complex. We find that damaging noise leads to nuclear AMPK activity specifically in inner hair cells and that Prkag2 deficient mice are susceptible to NIHL due to greater instability of the inner hair cell presynaptic ribbon. There is an urgent need to identify directed therapies aimed at the prevention and/or repair of cochlear damage from noise exposure, for which an understanding of the underlying mechanisms is an obligate prerequisite. Toward the long-term goal of developing targeted therapies for the prevention and/or correction of noise-induced synaptopathy, we now seek to decipher the pathways and mechanisms linking nuclear AMPK activity in inner hair cells to NIHL. Based upon our preliminary data, our central hypothesis is that AMPK becomes activated and trapped in the nucleus of inner but not outer hair cells by intranuclear phosphorylation after noise exposure and subsequently regulates the expression of downstream targets that impact the number and volume of presynaptic ribbons. Using a combination of genetics, physiology, cell biology, biochemistry, and structural biology, we propose the following three aims: the identification of cellular factors associated with susceptibility to NIHL (Aim 1), the molecular basis of nucleocytoplasmic shuttling of AMPK (Aim 2), and the identification of additional factors in the AMPK pathway leading to susceptibility to NIHL (Aim 3). As the AMPK pathway is fundamental to cell survival, metabolism, gene regulation, and hearing, and is targetable, the completion of these aims has the potential to lead to meaningful interventions for this debilitating condition.
Age-related hearing impairment (ARHI)
Age-related hearing impairment (ARHI) is the most common cause of hearing loss, is heritable, and is one of the most prevalent conditions affecting the elderly globally. Twin and family studies reveal 25-75% heritability for ARHI (Momi et al., 2015). Estimates suggest approximately two‐thirds of people over the age of 70 in the United States experience ARHI, and that by 2020, over half of all people in the United States with hearing loss will be over 70 years of age (Bainbridge and Wallhagen, 2014). ARHI has been shown to be independently associated with cognitive decline, dementia, depression, and loneliness and results in an estimated annual economic burden of over $3 billion (Deal et al., 2017; Deal et al., 2018; Lin and Albert, 2014). Our overarching hypothesis, supported by preliminary data in both mice and humans, is that ARHI is a complex trait with many likely genes associated (Fransen et al., 2015; Friedman et al., 2009; Kalra et al., 2019; Wells et al., 2019). Greater than 100 genes have been identified for monogenic deafness; however, a substantial fraction of patients with ARHI have no identifiable mutation in any known deafness gene suggesting that there remain additional genes to discover (Bowl and Dawson, 2018). Mice continue to be the predominant organism for hearing research. Similarities in the auditory structure and physiology between mice and humans, the close evolutionary relationship of genomes (most genes in mice have a human homologue), relatively low housing costs, genetic standardization and the available genetic toolkit make the mouse a crucial model system for the study of the functional genomics of the auditory system (Bowl and Dawson, 2015). We are proposing to identify candidate genes by performing the first complete genome-wide association study (GWAS) of ARHI in CFW mice and examining gene expression in the inner ear. Although several labs including ours have used human subjects for GWAS, to date there exist no comprehensively characterized cohorts with sufficient power and therefore there exist limited replication studies of candidate genes. In Aim 1, we will measure auditory brainstem response and distortion product otoacoustic emission thresholds in 2,000 one-year-old CFW mice equally divided among males and females. We will genotype each mouse at more than 1,000,000 single nucleotide polymorphism markers and in Aim 2 perform GWAS to identify quantitative trait loci (QTL). In Aim 3, we will use RNAseq to assess differences in gene expression in the inner ears of 100 randomly selected mice from the CFW cohort and define expressed QTLs (eQTLs).The genetic variation within CFW mice presents a unique opportunity to elucidate the molecular mechanisms that underlie ARHI providing novel targets for drug development and providing a means for identifying patients at risk.
Fall-related injury in the elderly
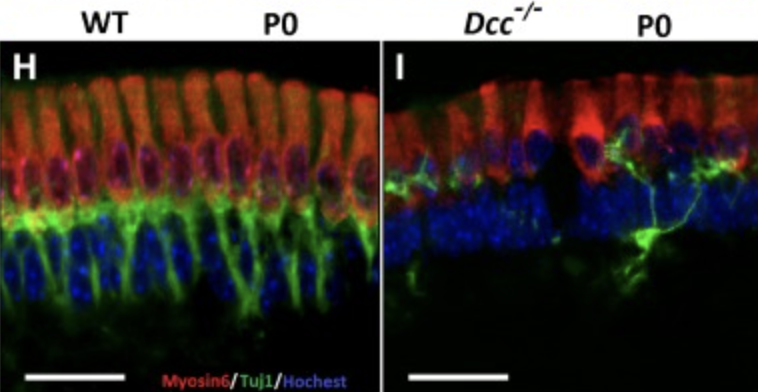
Fall-related injury in the elderly carries a 20% mortality rate, and is the sixth leading cause of death in this population. Age-related dysfunction of gravity receptors within the vestibular system is highly correlated with these elderly falls, and significant age-related degeneration is associated with nearly all types of vestibular cells. The overarching goal of this study is to analyze human and mice genome-wide association studies (GWAS), vestibular-specific human and mice genomic expression, and single-cell sequencing of specific sites in the vestibular system, then test identified genes and related pathways in the lab. Our objective is to characterize the genomics related to age-related imbalance for future prevention and treatment. The central hypothesis is that by this analysis, we can identify anatomic and physiologic sites relevant to the balance system that is common to both species. Our rationale is that by this analysis, we can better focus on relevant genes and pathways for lab testing and ultimate therapeutic intervention. Building upon a small GWAS on elderly falls that correlated human DCC and PTK2 genes in the same pathway as Dcc identified in the Hybrid Mouse Diversity Panel (HMDP) GWAS, our specific aims will be: 1) a. Perform GWAS in humans based on a dizziness/falls phenotype in a meta-analysis of large datasets; b. GWAS in mice based on a behavioral and gravity sensor function phenotype in the HMDP; 2) a. Perform RNA-Sequencing on vestibular tissues from mice and human surgical specimens; b. Single-cell RNA-Seq on individual tissues; c. Compare identified genes and pathways via computational methods to assess translation of pathways from the mouse to human balance system; and 3) Perform functional testing for the top candidates defined in Aim 2 using knock-out/knock-in mice. Multiple innovations of this project include: 1) the first GWAS of gravity receptor function in aged mice and in elderly humans, 2) a comprehensive catalogue of genes and pathways involved in vestibular functional variation with inter-species comparison, as part of FAIR (findable, accessible, interoperable, reusable) Compliance, 3) in vivo validation in mouse models and an analysis of these candidates in available human cohorts, and 4) future potential for targeted therapies. Our outcome is the first comparative GWAS of the balance system between animal models and humans. The impact of this work will be to lay a firm foundation for development of targeted treatment of the balance system to diminish falls in the elderly.
